CARDIOPATCH's Therapy Model
CARDIOPATCH advanced therapy model for MI treatment is based in two promising fields: cellular regenerative medicine and 3D printing technology. The aim is to create a definitive therapeutic solution that can benefit patients with chronic myocardial infarction significantly allowing them to recover from their disease without the need of long-term pharmacological treatments or further hospitalizations.
Cardiac tissue regeneration with preconditioned ADSC, iPS-CM and/or their exosomes in combination with tissue engineering seems promising therapeutic options that deserve to be investigated. Yet, cell-therapy studies for heart disease have not achieved their clinical goals so far, likely because of the lack of studies on patient-mimicking pig animal models presenting with cardiovascular risk factors/co-morbidities.
CARDIOPATCH wants to move to a new stage in this research field by taking the advantages that 3D printing technology offers.
Cardioreg patch and health LSR silicone frame
CARDIOPATCH advanced therapy takes as starting points two products developed in the frame of two European projects: CARDIOREG (nº2019/3) and HEALTH LSR (EFA0003/15).
The goal is to capitalise these two products which means, transfer the knowledge gained in their generation to the lab activities and on the base of new needs, transform them in improved products which will compose the CARDIOPATCH advanced therapy for the MI treatment.
CARDIOREG patch
In 2010, UNAV and FIMA started producing collagen patches as cell support. In collaboration with the company VISCOFAN, and in the frame of CARDIOREG project (Nº2019/3) approved by AECT Eurorregión Nueva Aquitania – Euskadi – Navarra, these entities developed a collagen patch, biodegradable and celluralized with ADSC, in compliance with the GMP (Good Manufacturing Practice) requirements, which will allow its rapid commercialization and use in patients. This patch is known as CARDIOREG Patch. A Phase I clinical trial has already begun in patients with ischemic heart disease.
HEALTH LSR- silicone frame
Patent: Request number EP19382250.9
Title: Devices for cellularized membrane cultivation and kits
This device was developed by LEARTIKER and the University Clinic of Navarra in the frame of the HEALTH LSR project (EFA0003/15) in 2019.
Characteristics:
- Watertight device.
- It withstands well an internal overpressure of 2.7 times its volume without losing hermeticity.
- Nice fizzy swap.
- It allows maintaining the sterility of the cultures. Transport under GMP conditions of the tissue to the operating room.
- The fluid exchange system through the Luer-Lock ports is efficient and keeps the system sterile.
- Device compatible with different types of membranes.
- The device meets the requirements and allows the growth of at least the tested cell line.
Applicable in:
- Tissue engineering: 3D cultures on membranes.
- Dermatology: Artificial skin, melanocytes for vitiligo.
- Ophthalmology: Limbocorneal cells.
- Urology: cellularized membranes for pelvic floor.
- Cardiology: Cellularized Membranes for Myocardial Infarction.
Capitalisation process
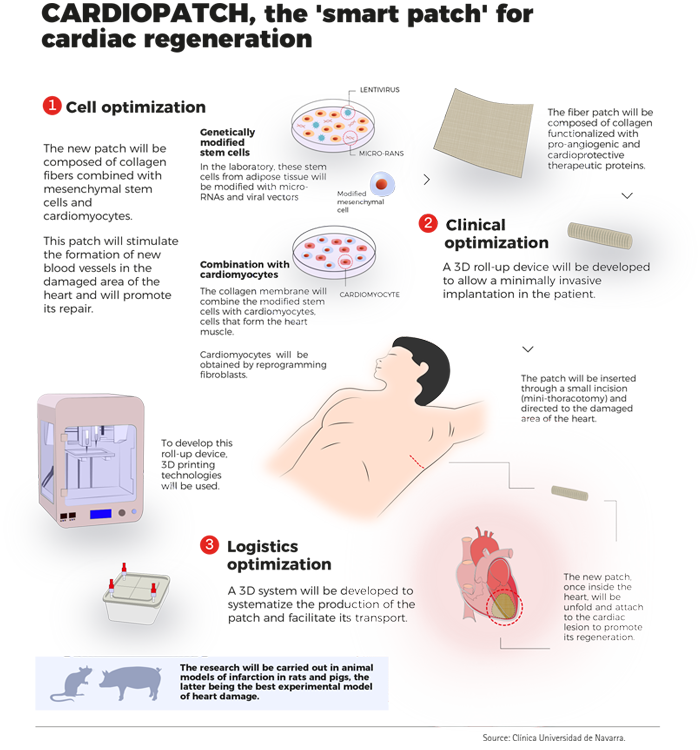
- Create a 2.0 version patch (v2.0) with growth factors and genetically improved mesenchymal cells and iPS-derived cardiac cells that improves cell survival of both the implanted cells and the ischemic cardiac tissue as well as their pro-angiogenic capacity.
- Create a 3D device for its culture and transport.
- Improve the method of implanting the patch so that it can be performed in a less invasive manner.
Lab activities & Final Products
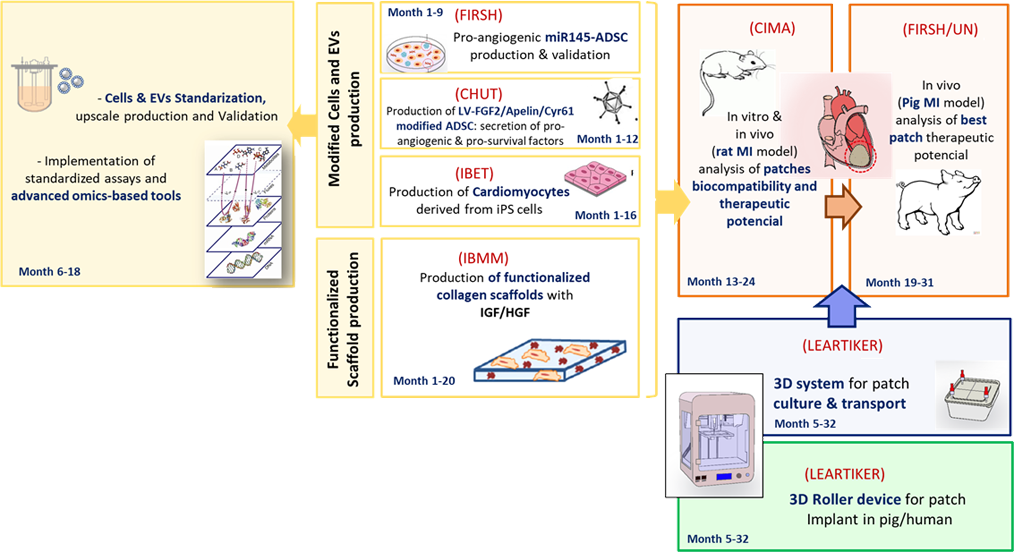
The project members will develop therapeutic patches (v2.0) prepared with improved cells and functionalized membranes. Adipose-derived mesenchymal stem cells (ADSC) will be genetically modified by the Research Institute of the Hospital de la Santa Creu i Sant Pau (HSCSP-IR) and the Toulouse Hospital (CHUT) in order to increase their pro-angiogeneic and cardioprotective trophic effect into the heart. Moreover, ADSC will be combined with cardiomyocytes previously derived from pluripotent stem cells (iPSC) by the Instituto de Biología Experimental e tecnología (iBET), in order to increment the regenerative properties of the patch. Cells and biological derivatives such as exosomes will be validated by an array of omics-strategies in order to obtain the best therapeutic cellular products that can be applied to the patients in the future.
This task will be performed by iBET and Genibet Biopharmaceuticals. Furthermore, the collagen scaffold will be functionalized with IGF and HGF cytokines, whose cardioprotective and pro-angiogeneic effect has been also previously shown. Use of functionalized patches (prepared by Université de Montpellier- Institut des biomolecules Max Mousseron (IBMM) will allow a controlled and sustained release of the therapeutic growth factors in the damaged area of the heart. Thus, the different patches will be validated in vivo in rodent and pig models of myocardial infarction (Center for Applied Medical Research, HSCSP-IR and University of Navarra) in order to confirm their putative therapeutic potential.
On the other hand, new designs of 3D-system prototypes for the patches preparation, transport and non-invasive implantation into the heart will be designed as a model of cellular regenerative therapy aimed at patients with chronic heart disease (Leartiker).
The final products that will compose CARDIOPATCH Therapy Model will be:
- Cellularized and Functionalized patches.
- Culture and Transport patch device.
- 3D Roll-up device for patch non-invasive implantation.